Beer Drinking and Development of Physical Dependence
Md S. Zaman and Francine E. Lancaster
Department of Biological Sciences, Alcorn State University, Lorman, MS 39096, and
Department of Biology, Texas Woman's University, Denton, TX 76204
Beer, an alcoholic beverage and popular drug of abuse in our society, is known to be hazardous from medical and social view points. Although alcohol content in beer is low, chronic beer consumption may lead to the development of alcohol dependence. To study beer drinking patterns and the development of physical dependence on alcohol contained in beer, Long Evans female rats were allowed to drink beer for 12 hours during the light cycle and 12 hours during the dark cycle. In order to study the development of physical dependence on alcohol, rats were administered beer for 21 days, receiving 24.43% of their total caloric intake from beer. The estimated ethanol consumption was approximately 5.98 g/kg/day. The animals consumed the highest quantity of beer during the first hour of each light and dark cycle. After the termination of ethanol treatment, various withdrawal signs occurred in rats, including tail stiffening, piloerection, hypoactivity, hyperactivity, shivering, and hypothermia. These withdrawal symptoms indicated development of physical dependence on alcohol in beer treated animals.
Key Words: Beer, alcohol withdrawal, hypothermia, physical dependence.
Alcohol is a popular drug of abuse in our society, and alcoholism is an important cause of major health and social problems (Gianoulakis, 1993; Rabbia et al., 1995). Alcoholism is a growing problem, and at the present time, it is recognized as a metabolic disease exhibiting the features of craving for alcohol, tolerance to and physical dependence on alcohol (Gianoulakis and deWaele, 1994). Alcoholism affects people of all ages, races, sexes, and social status. An alcoholic sub-group that has been increasingly scrutinized in the areas of academic research and clinical treatment is that of alcoholic women (Hochgraf et al., 1995).
When humans or animals chronically consume alcohol, a series of behavioral and physiological disturbances are observed upon abrupt withdrawal of alcohol. These withdrawal syndromes are interpreted as physical dependence on alcohol. Withdrawal syndromes are thought to be due to adaptations by CNS neurons to the presence of the drug of abuse (Whittington et al., 1995).
Withdrawal reactions have been defined as a group of signs and symptoms that develop after withdrawal and physical dependence as a biochemical or biophysical abnormality that develops progressively during chronic alcohol administration (Goldstein, 1975). Cellular or molecular mechanisms involved in ethanol tolerance and dependence are not well understood (Mhatre et al., 1993; Boyadjieva and Sarker, 1994). Since the intensity of physical dependence is difficult to measure at cellular levels, the measurement of dependence is based on the degree of intensity of the withdrawal behavior.
In many of the previous alcohol studies where animal models have been used to measure the development of physical dependence, the intake of ethanol was obligatory (Branchey et al., 1971; Ritzmann and Tabakoff, 1976; Suzuki et al., 1992; Ulrichsen et al., 1992; Pohorecky and Roberts, 1992). Voluntary consumption of ethanol along with normal diet may be important in the study of development of physical dependence, because self administration of alcohol by rats may mimic human drinking patterns.
Beer is a popular alcoholic beverage in the USA, Australia, and most European countries. Although beer is rich in nutrients and vitamins, it is hazardous from medical view points because of its alcohol content. Alcohol is known to produce neurologic, cardiovascular, renal, hepatic, developmental, and other abnormalities (Karthigasu, 1970; Kaminski et al., 1981; Kuzma and Sokol, 1982; Potter and McMichael, 1984; Lancaster et al., 1989; Bouchardy et al., 1990; Graham et al., 1990; Riboli et al., 1991; Harper and Kril, 1992; Dodd et al., 1992; Butterworth et al., 1993; Costello et al., 1993; Mannella and Beauchamp, 1993; Pehl et al., 1993; Ades and Lejoyeus, 1994).
Since alcohol content in beer is low (35%), most people do not suspect that chronic beer
consumption may lead to the development of alcohol dependence. In this study, we are reporting
beer drinking patterns (Lancaster et al., 1987), and development of physical dependence on the
alcohol contained in beer following voluntary, chronic beer consumption by female rats as
indicated by withdrawal syndromes.
MATERIALS AND METHODS
Animals and housing--Adult Long Evans female rats were housed individually in transparent plastic cages with stainless steel tops. Lighting was regulated to 12 h light and 12 h dark. A constant room temperature of 22C was maintained throughout the study period. All animals were allowed ad libitum access to food (Purina Lab chow with a caloric value of approximately 3.92 kcal/g) and water throughout the study period. In addition to regular diet and water, beer drinking animals were allowed ad libitum access to Budweiser beer containing 5% alcohol and caloric value of approximately 0.43 kcal/ml. (Anheuser-Busch, Inc., St. Louis, MO, USA). Beer, food, and water consumption was measured daily and the rats' body weights were measured at the beginning and at the end of the study.
Pattern of beer drinking for a 12 h period of dark and 12 h period of light--Eighteen adult female Long Evans rats that had no previous experience with beer were maintained on the light and dark cycles for about one week prior to beer drinking pattern experiments. Animals were allowed to drink beer (Budweiser) for a 12 h period of light and 12 h period of dark. Beer was given to the rats in calibrated feeding tubes. Consumption was recorded every hour. Drinking under light and dark conditions was studied on separate days to provide a rest interval (one week) between the two experiments.
Development of physical dependence in rats by voluntary, chronic beer consumption--These experiments were conducted one week after drinking pattern studies. Sixteen rats from drinking pattern studies were used for dependence experiments. Rats were divided into two groups, a beer group (N=8) and a control group (N=8). Animals that consumed highest quantities of beer in drinking pattern study were selected for beer group. Animals that showed least interest in beer drinking were selected for control group. Beer treated animals were allowed ad libitum access to beer for 21 days along with their normal diet and water. Control animals had no access to beer, but were given food and water ad libitum. At the end of the 21st day of beer administration, beer was withdrawn; thereafter, the animals were observed for a number of withdrawal syndromes (hypothermia, tail stiffening, piloerection, tail arching, hypoactivity, hyperactivity, spontaneous vocalization, whole body rigidity, tremors) for a period of 8 hours. Presence of any of these signs was used to indicate physical dependence on alcohol (Walker et al., 1975). Withdrawal syndromes were studied during the light cycle (8:00 A.M. to 4:00 P.M.)
Body temperature was measured at the beginning of the withdrawal period (rectal temperature was taken by 'Astrotemp-9' electronic digital thermometer; Marshall Electric Inc., IL, USA), and then on the 2nd, 4th, 6th, and 8th hours of the withdrawal period.
Statistical analyses--Data were analyzed by analysis of variance (ANOVA). Significant 'F' ratios
were evaluated by Newman Keul's multiple range test.
RESULTS
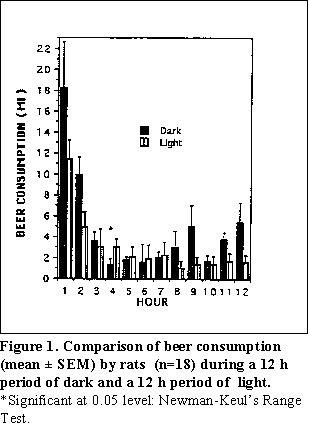
Pattern of beer drinking for a 12 h period of dark and 12 h period of light--During the first hour of light or dark. the animals consumed the highest quantities of beer (Fig. 1). Mean beer consumption for each hour during the light was 2.89 ± 1.15 ml, while the mean beer consumption for each hour during the dark was 4.97 ± 1.47 ml. A significant difference in beer consumption between the hours of dark versus light was observed only during the fourth hour of consumption (p < 0.01) (Fig. 1).
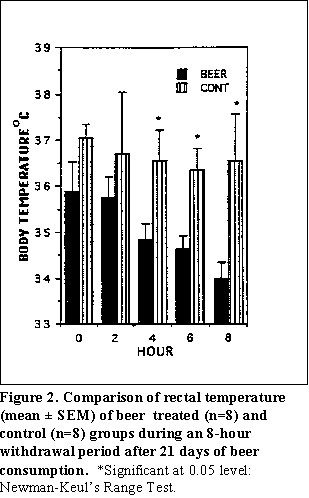
Development of physical dependence in rats by voluntary, chronic beer consumption--After the 21st day of beer administration, beer was withdrawn and withdrawal symptoms were observed. At the beginning of the withdrawal period, mean body temperature was 35.9 ± 0.6C for the beer treated animals and 37.4 ± 0.3C for the control animals. Significant differences in body temperature between the control and beer treated animals were observed during the fourth (p0.02), sixth (p0.006) and eighth (p0.004) hours of alcohol withdrawal period (Fig. 2).
Among the beer treated animals, tail
stiffening was observed in five (62.5%);
piloerection was observed in four (50%);
hypoactivity was observed in four (50%);
hyperactivity was observed in three (37.5%);
and shivering was observed in three (37.5%).
All the treated animals presented some of
these withdrawal symptoms. None of the
beer treated animals showed tail arching,
spontaneous vocalization, whole body
rigidity, or tremors. None of these
withdrawal behaviors were observed in
control animals.
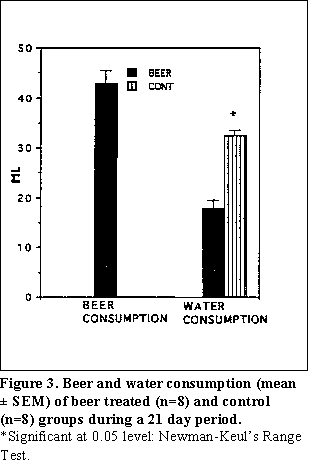
Beer consumption--During the 21 days of beer administration, the highest mean beer consumption (ml beer/rat) was 62.63 ± 8.00 ml and the lowest mean beer consumption was 24.67 ± 11.00 ml on day 20 and day 7 respectively. The mean, beer consumption for 21 days was 42.80 ± 2.62 ml (Fig. 3). The estimated mean ethanol consumption from beer for 21 days was 5.9 g/kg/day.
Water consumption--The mean water consumption (ml water/rat/day) for 21 days was 18.21 ± 1.69 ml and 32.96 ± 0.92 ml for the beer group and the control group respectively. The mean water consumption by the control group was significantly higher than that of the beer group (Figure 3). The total water consumption (regular water plus the water content of the beer) by the beer group was significantly higher than the control group on each day except for day 7. The mean 'total water' consumptions for 21 days were 59.24 ± 2.21 ml and 32.96 ± 0.92 ml for the beer group and for the control group respectively.
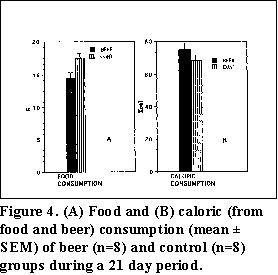
Food and caloric consumption--Mean food consumption (g food/rat/day) for 21 days was slightly higher in the control group than in the beer group; values were 17.51 ± 0.79 g and 14.44 ± 0.88 g respectively (Fig. 4). Mean caloric consumption (from food and beer) for 21 days was 75.10 ± 3.90 kcal for the beer group and 68.62 ± 3.09 kcal for the control group (Fig. 4).
Body weight--The mean body weight of all the animals (control and beer treated in the dependence study) at the beginning of the study was 282.50 ± 10.00 g. At the end of the study, the mean body weights were 286.00 ± 8.57 g for the control animals and 287.13 ± 11.13 g for the beer treated animals. Therefore, there was no significant increase in body weight during the study period, and there were no differences in the weights of the control and beer groups.
DISCUSSION
The concept of addiction includes tolerance and physical dependence (Sees and Clark, 1994). Tolerance to a drug may be defined as the need to increase the amount of drug to maintain a desired effect (Ziolkowski et al., 1995), and physical dependence can be detected by observing withdrawal symptoms. Results from the present study suggest that chronic beer consumption for 21 days in amounts of 42.80 ± 2.62 ml can develop physical dependence in adult female Long Evans rats. Walker et al. (1975) classified the alcohol withdrawal syndromes in rats into two major categories, the pre-convulsive stage and convulsive stage. These two stages were again sub-divided into seven stages according to the progress of the severity of the withdrawal syndromes, which started with tail stiffening and ended with convulsion terminating in death. The withdrawal symptoms observed in the current study included the signs of Stage I (tail stiffening and piloerection) and Stage 3 (hypoactivity and hyperactivity). Shivering was also observed. All the treated animals presented some of these withdrawal symptoms. These signs are considered as mild to moderate withdrawal symptomology (Friedman, 1980).
Hypothermia, a parameter of physical dependence, was observed in the treated group during the alcohol withdrawal period (Fig. 2). Significant differences in body temperature between the control and beer treated animals were observed during the fourth, sixth, and eighth hours of the alcohol withdrawal period. In another study, we also observed development of hypothermia in rats during the fourth and sixth hours of beer withdrawal period following chronic beer consumption (Lancaster et al., 1987).
Previously, it was reported that administration of 7.6 g/kg/day of ethanol produced hypothermia in mice during withdrawal at 20C and 25C environmental temperatures (Malcolm and Alkana, 1983). Previously, we also reported that administration of 11.0 g/kg/day alcohol at 22C would produce hypothermia in rats during withdrawal (Lancaster et al., 1987). Results from the current study indicate that administration of ethanol in the quantity of as little as 5.9 g/kg/day at 22C would also produce hypothermia in rats.
Several studies indicated that in ethanol dependent rats the mean alcohol consumption was between 6.012.0 g/kg/day (Branchey et al., 1971; Malcolm and Alkana, 1983; Pohorecky and Roberts, 1992; and Suzuki et al., 1992). Results from the current study indicate that ethanol consumption in amounts of 5.9 g/kg/day over a period of 21 days will produce mild to moderate ethanol dependence in rats.
Although the mean food consumption for 21 days was higher in the control group, the beer group consumed more calories which were derived from beer. Therefore, the beer group did not have decreased caloric intake, but probably did have decreased nutrient intake.
The drinking pattern study showed that during the first hour of beer administration of light or dark cycles, rats consumed the maximum amount of beer. This finding is consistent with our previous finding (Lancaster et al., 1987). In the previous study, rats consumed more beer during the light cycle, while in the current study rats consumed more beer during the dark cycle. This result suggests that although rats are nocturnal animals, dark or light phases had little or no effect on the drinking behavior of rats. Animals consumed beer whenever it was available. Consumption of beer in large quantities in a single bout has also been observed in humans. A sudden drop in quantities of beer consumption in subsequent hours during dark or light cycle was probably due to satiation from beer intake by the animals.
Female rats in this study received 24.43% of their total caloric intake from beer (estimated mean
daily caloric intake from food = 14.44 kcal, from beer = 18.50 kcal). In a woman, this amount is
equivalent to drinking about 3.5 cans (355 ml each) of regular beer per day. Beer consumption by
female rats over a period of 21 days is equivalent to beer consumption by women over a period of
about 22 months. Since 24.43% of caloric consumption from beer over a period of 21 days
produced physical dependence in female rats, it may be suggested that drinking 3.5 cans of beer
per day for a period of 22 months may contribute to the development of physical dependence in
women. These calculations were derived considering 2,200 kcal as the daily caloric consumption
for an average woman, 79.1 years as the average life expectancy of women in U.S. (Kochanek et
al., 1995), and 2.5 years as the average life expectancy of Long Evans rats according to Harlan
Sprague Dawley, Inc. (Indianapolis, IN, USA). Body weight ratio of rats and humans were not
considered in this estimation, since the caloric requirement per unit body weight in rats is much
higher than that in humans.
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr. Sumit Mukhopadhyay, Purdue University, West
Lafayette, Indiana, Drs. Abulkhair M. Mamoon and Naila M. Mamoon, University of Mississippi
Medical Center, Jackson, Mississippi, and Ms. Fahmida Zereen, Alcorn State University, Lorman,
Mississippi, for their support.
LITERATURE CITED
Ades, J., and M. Lejoyeus. 1994. Addictive behavior in the elderly. Revue du praticien. 44:14391442.
Bouchardy, C., F. Clavel, C.L. Vecchia, L. Raymond, and P. Boyle. 1990. Alcohol, beer and cancer of the pancreas. Int. J. Cancer. 45:842846.
Boyadjieva, N.I., and D.K. Sarker. 1994. Effects of chronic alcohol on immunoreactive beta-endorphin secretion from hypothalamic neurons in primary cultures: evidence for alcohol tolerance, withdrawal, and sensitization responses. Alcohol. Clin. Exp. Res. 18:14971501.
Branchey, M., G. Rauscher, and B. Kissin. 1971. Modifications in the response to alcohol following the establishment of physical dependence. Psychopharmacology. 22:314322.
Butterworth, R.F., J.J. Kril, and C.G. Harper. 1993. Thiamine-dependent enzyme changes in the brains of alcoholics--relationship to the Wernicke-Korsakoff-syndrome. Alcohol. Clin. Exp. Res. 17:10841088.
Costello, R., A. Huissoon, and W.T. McNicholas. 1993. Multifactorial hypokalemia in a beer drinker. In J. Med. Sci. 162: 263264.
Dodd, P.R., G.J. Thomas, C.G. Harper, and J.J. Kril. 1992. Amino-acid neurotransmitter receptor changes in cerebral-cortex in alcoholism--effect of cirrhosis of the liver. J. Neurochem. 59:15061515.
Friedman, H. J. 1980. Assessment of physical dependence on and withdrawal from ethanol in animals. Pages 93121 in H. Rigter and J.C. Crabbe, Jr. (eds.), Alcohol Tolerance and Dependence. Elsevier/North Holland Biomedical Press, Amsterdam, New York, Oxford.
Gianoulakis, C. 1993. Endogenous opioids and excessive alcohol consumption. J. Psychiat. Neurosci. 18:148156.
Gianoulakis, C., and J.P. deWaele. 1994. Genetics of alcoholism: role of the endogenous opioid system. Metabol. Brain Dis. 9:105131.
Goldstein, D.B. 1975. Pharmacological aspects of physical dependence on ethanol. Life Sci. 18:553562.
Graham, S., J. Marshall, B. Haughey, J. Brasure, J. Freudenheim, M. Zielezny, G. Wilkinson, and J. Nolan. 1990. Nutritional epidemiology of cancer of the esophagus. Am. J. Epidemiol. 131:454467.
Harper, C.G., and J.J. Kril. 1992. If you drink your brain will shrink. Alcohol Alcoholism. suppl 1: 375380.
Hochgraf, P.B., M.L. Zilberman, and A.G.D. Andrade. 1995. Women alcoholics--Social, demographic and clinical characteristics in a Brazilian sample. Alcohol Alcoholism. 30:427432.
Kaminski, M., M. Franc, M. LeBouvier, C. du Mazaubrun, and C. Rumeau-Rouquette. 1981. Moderate alcohol use and pregnancy outcome. Neurobehav. Toxicol. Teratol. 3:173181.
Karthigasu, M. 1970. Beer drinker's cardiomyopathy, liver failure, hypothermia, hypo-osmolality, inappropriate secretion of antidiuretic hormone, lactic acidosis, venous gangrene and myoneuropathy. Proc. Royal Soc. Med. 70:544548.
Kochanek, K.D., M.A. Bettie, and L. Hudson. 1995. Advanced report of final monthly statistics, 1992. Monthly Vital Statistics Report. 43 (6): National Center for Health Statistics, U.S. Department of Health and Human Services. Publication No. (PHS) 95-1120.
Kuzma, J. W., and R.J. Sokol. 1982. Maternal drinking behavior and decreased intrauterine growth. Alcohol. Clin. Exp. Res. 6:396402.
Lancaster, F., K. Spiegel, and M.S. Zaman. 1987. Voluntary beer drinking in rats. Alcohol Drug Res. 7:393403.
Lancaster, F.E., Z.A. Raheem, and K. Spiegel. 1989. Maternal beer drinking: offspring growth and brain myelination. Neurotoxicology. 10:407416.
Malcolm, R.D., and R.L. Alkana. 1983. Temperature dependence of ethanol lethality in mice. J. Pharm. Pharmacol. 35:306311.
Mannella, J.A., and G.K. Beauchamp. 1993. Effects of beer on breast-fed infants. JAMA. 269:16371638.
Mhatre, M.C., G. Pena, W. Sieghart, and M.K. Ticku. 1993. Antibodies specific for GABAA receptor alpha subunits reveal that chronic alcohol treatment down-regulates alpha-subunit expression in rat brain regions. J. Neurochem. 61:16201625.
Pehl, C., B. Wendl, A. Pfeiffer, T. Schmidt, and H. Kaess. 1993. Lowproof alcoholic beverages and gastro-esophageal reflux. Dig. Dis. Sci. 38:9396.
Pohorecky, L.A,. and P. Roberts. 1992. Daily dose of ethanol and the development and decay of acute and chronic tolerance and physical dependence in rats. Pharmacol. Biochem. Behav. 42:831842.
Potter, J.D., and A.J. McMichael. 1984. Alcohol, beer and lung cancer, a meaningful relationship? Int. J. Epidemiol. 13:240242.
Rabbia, F., F. Veglio, R. Russo, D. Schiavone, S. Oliva, and L. Chiandussi. 1995. Role of alcoholic beverages in essential hypertensive patients. Alcohol Alcoholism. 30:433439.
Riboli, E., J. Cornee, G.M. Moulin, R. Kaaks, C. Casagrande, and M. Guyader. 1991. Cancer and polyps of the colorectum and lifetime consumption of beer and other alcoholic beverages. Am. J. Epidemiol. 134:157166.
Ritzmann, R.F., and B. Tabakoff. 1976. Body temperature in mice: quantitative measure of alcohol tolerance and physical dependence. J. Pharmacol. Exp. Ther. 199:158170.
Sees, K.L., and C.H.W. Clark. 1994. Opiold use in the treatment of chronic pain: assessment of addiction. J. Pain Symp. Manage. 8:257264.
Suzuki, T., H. Motegi, K. Otani, Y. Kolke, and M. Misawa. 1992. Susceptibility to, tolerance to, and physical dependence on ethanol and barbital in two inbred strains of rats. Gen. Pharmacol. 23:117.
Ulrichsen, J., L. Clemmesen, and R. Hemmingesen. 1992. Convulsive behavior during alcohol dependence: discrimination between the role of intoxication and withdrawal. Psychopharmacology. 107:97102.
Walker, D.W., B.E. Hunter, and J. Riley. 1975. A behavioral and electrophysiological analysis of ethanol dependence in the rat. Pages 353372 in M. M. Gross (ed.), Advances in Experimental Medicine and Biology--Alcohol Intoxication and Withdrawal. Plenum Press, New York, London.
Whittington. M.A., J.D.C. Lambert, and H.J. Little. 1995. Increased NMDA receptor and calcium channel activity underlying ethanol withdrawal hyperexcitability. Alcohol Alcoholism. 30:105114.
Ziolkowski, M., E. Maludzinska, T. Gruss, J. Rybakowski, and J.R. Volpicelli. 1995. Decrease in alcohol tolerance: clinical significance in alcohol dependence. Drug Alcohol Depend. 39:3336.